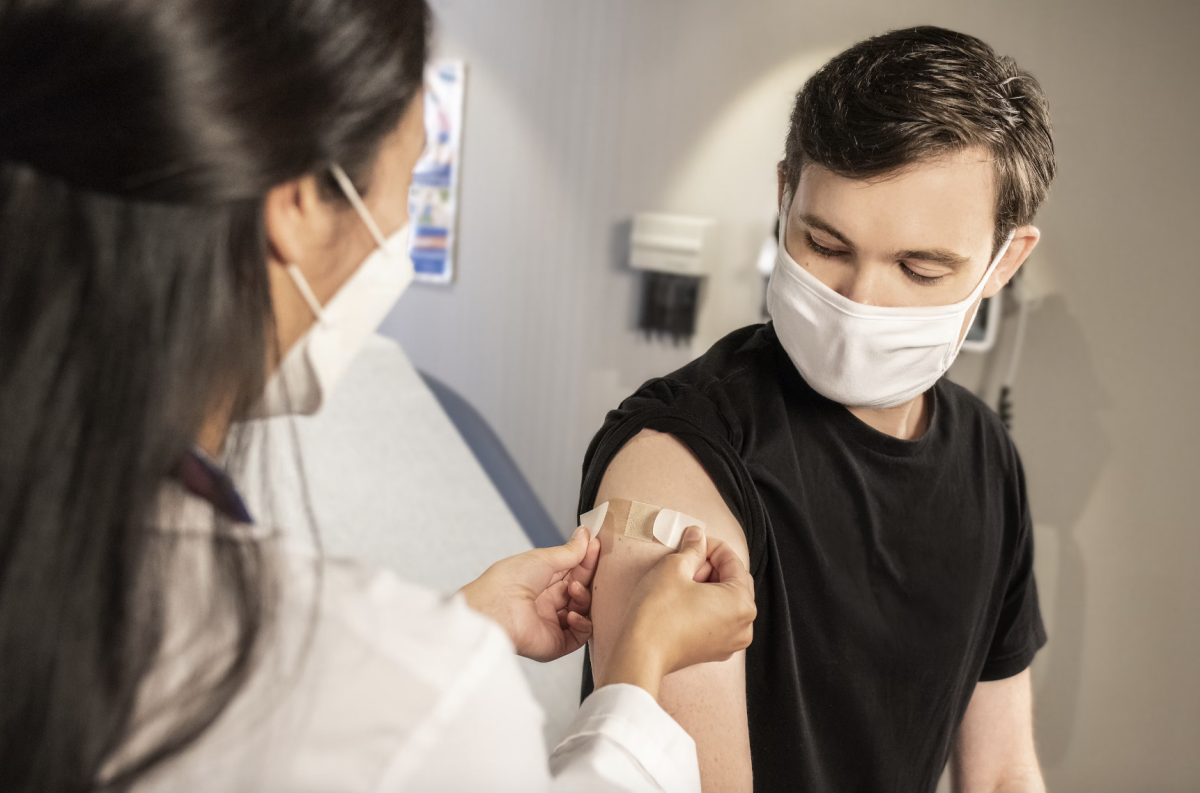
The Food and Drug Administration (FDA) and the Center for Disease Control and Prevention (CDC) approved the latest COVID-19 vaccine to be administered by Pfizer-BioNtech, Moderna, and Novavax. The boosters are intended to prevent the spread of new omicron sub-variants of the virus, incuding XBB1.5, as well as other strands of the virus such as EG.5 and BA2.86 in late September.
Experts say that new sub-variants spread more rapidly and have milder effects than its predecessors. The virus is also easier to contract, and COVID-19 tests may continue to display as positive even as symptoms subside.
The CDC began to recommend the new COVID-19 booster on Sept. 12. Since Oct. 11, over 7 million Americans in the United States have received the updated COVID-19 booster, according to data from the United States Department of Health and Human Services. However, some reports indicate that people are struggling to find vaccination appointments in pharmacies in well-populated areas with high demand for the new booster.
North Carolina contributes an average of 60 cases per day to the nearly 20,000 new COVID-19 cases reported daily in the United States.
Some question the efficacy of the new boosters, as a majority of people recorded to contract the virus are vaccinated.
The CDC and FDA also encourage young adults and children older than six months to get the booster, as the virus has caused over 200,000 deaths since Jan. 2022. Among the people who have died from the virus, 600 were under the age of 19.
Older people are also advised to receive the vaccine. People over the age of 50 are at risk of contracting further infections vulnerable when infected with the virus. These issues may also lead to long-term implications, such as chronic illness and immunodeficiency, declining the immune system’s responsiveness.
Certain recipients of prior COVID-19 vaccines question whether they can safely receive vaccines produced by companies different than the company that produced their initial vaccine dose due to reports of women aged 18 to 48 reportedly being diagnosed with rare blood clots after taking the Johnson & Johnson vaccine.
As new boosters continue to roll out, their effects remain to be seen.